Studieoverzicht
Study name: DIRECT
| Histology | NSCLC, all subtypes | ||
|---|---|---|---|
| Tumor stage | Stage I - III | ||
| Host / recruiting site 1 | Amsterdam UMC | Enrollment | Closed |
| Therapy line | First line (1L) | ||
| Design |
A single center, single arm, phase 1 / 2 trial |
||
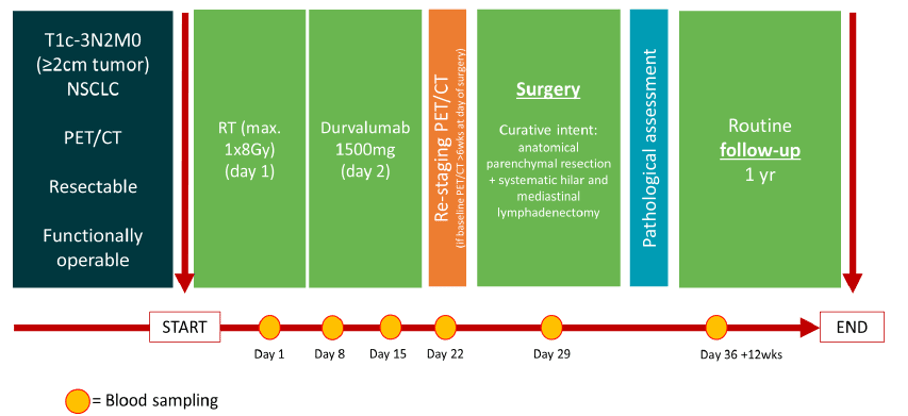
| Intervention | RT will be applied to the primary tumor in 1 fraction of 8Gy, followed by one dose of durvalumab. Resection is planned approximately 4 weeks after the RT and durvalumab administration. |
||
| Key outcome parameters | safety defined as the percentage of patients with adverse events. major pathological response (MPR) and pathological complete response (pCR) rates in resected tumor-specimens. tumor immune infiltration after durvalumab and RT in 4 categories radiological response to durvalumab and RT using RECIST v1.1 criteria, and metabolic response based on 18F-FDG PET |
||
| Key inclusion criteria | Histologically confirmed NSCLC T1c-3N0, here T3 tumors are based on size, but not based on invasion into the thoracic wall, mediastinum, vertebra or diaphragm or ipsilateral lung nodules Willing and able to provide written informed consent for the trial Above 18 years of age on day of signing informed consent Have measurable disease based on RECIST 1.1 Have a ECOG performance status of 0-1, and are considered operable based on pulmonary function test and/or exercise testing Demonstrate adequate organ function, as deemed acceptable by the treating physician in the context of immunotherapy |
||
| Key exclusion criteria | Prior surgery and/or radiotherapy on the ipsilateral thorax Patients deemed inoperable Subjects with a condition requiring systemic treatment with either corticosteroids (> 10 mg daily prednisone equivalent) or other immunosuppressive medications within 14 days of day 0. Inhaled or topical steroids, and adrenal replacement steroid >10 mg daily prednisone equivalent, are permitted in the absence of active autoimmune disease Additional malignancy that is progressing or requires active treatment. Exceptions include basal cell carcinoma of the skin, squamous cell carcinoma of the skin, or in situ cervical cancer that has undergone potentially curative therapy Active or prior documented autoimmune or inflammatory disorders (including inflammatory bowel disease [e.g., colitis or Crohn’s disease], diverticulitis [with the exception of diverticulosis], systemic lupus erythematosus, Sarcoidosis syndrome, or Wegener syndrome [granulomatosis with polyangiitis, Graves’ disease, rheumatoid arthritis, hypophysitis, uveitis, etc.]). The following are exceptions to this criterion:
|
||
| Contact information | Log in voor de contactinformatie | ||