Studieoverzicht
Study name: Drug Access Protocol (DAP)
| Histology | NSCLC, all subtypes | ||
|---|---|---|---|
| Tumor stage | Stage III - IV | ||
| Host / recruiting site 1 | Amsterdam UMC | Enrollment | Closed |
| Host / recruiting site 2 | Antoni van Leeuwenhoek | Enrollment | Recruiting |
| Host / recruiting site 3 | Erasmus MC | Enrollment | Recruiting |
| Host / recruiting site 4 | UMC Groningen | Enrollment | Recruiting |
| Host / recruiting site 5 | MUMC+ | Enrollment | Recruiting |
| Host / recruiting site 6 | Radboud UMC | Enrollment | Recruiting |
| Host / recruiting site 7 | LUMC | Enrollment | Recruiting |
| Design |
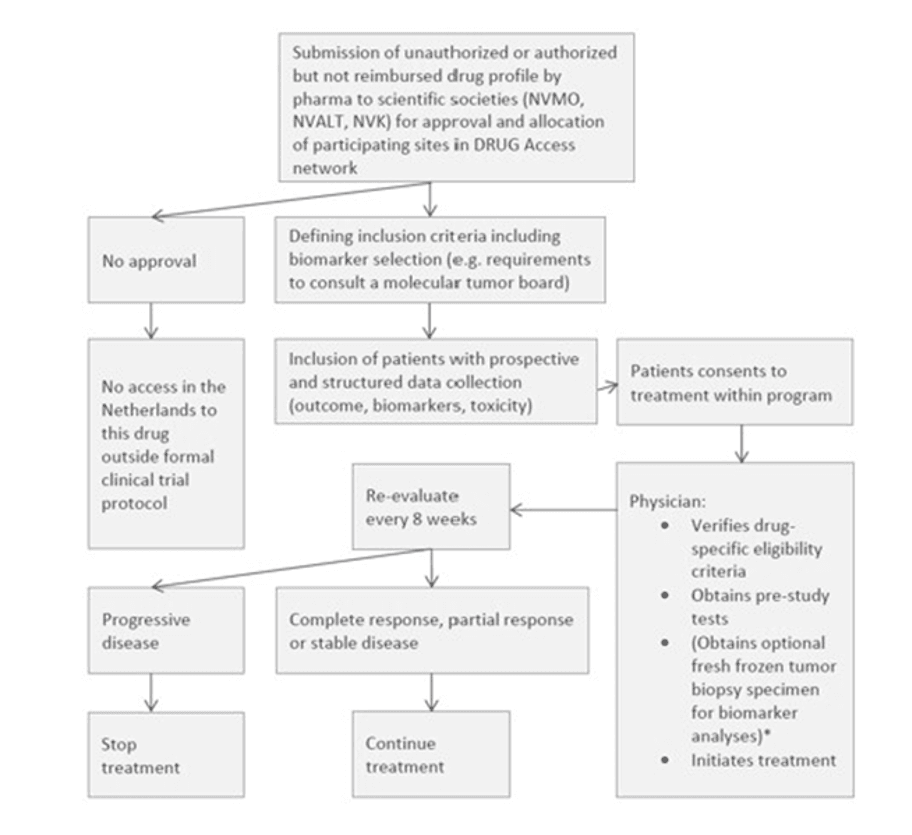
Prospective, open-label, non-randomized data collection trial. |
||
| Intervention | Based on the tumor characteristics, a targeted drug is made available.
|
||
| Key outcome parameters | Collect data on the effectiveness and safety of these anti-cancer drugs while they await reimbursement. |
||
| Key inclusion criteria | Solid cancer and acceptable performance status and organ function. For authorized indications or for drugs with a positive CHMP opinion, the eligibility and age restriction will be based on the (proposed) EMA label. If the drug specifically targets a molecular profile, a genomic or protein expression test must have been performed on the tumor and results must identify this potentially actionable molecular profile. Objectively evaluable or measurable disease (by physical or radiographic examination), according to RECIST v1.1 for patients with solid tumors. If a patient fulfils all drug-specific criteria but does not meet the criterion of measurable disease according to one of the above-mentioned evaluation protocols, patients can still be included in the study. In that case, method of treatment evaluation will be decided by the treating physician after consultation of central study team. If the drug specifically targets a molecular profile, results must be available from a tumor genomic or protein expression test. Eligible tests may include: FISH, PCR, CGH, NGS or IHC. The test results (full pathology or molecular diagnostics report) must be uploaded in the eCRF. Patients must have a tumor profile for which treatment with an unauthorized drug awaiting FDA/EMA approval or with an authorized anticancer drug that is awaiting reimbursement in the Netherlands has potential clinical benefit based on clinical data. In case patient agrees with obtaining a pre-treatment biopsy: a new (obtained ≤2 months before inclusion, and without any type of anti-cancer therapy within those ≤2 months) fresh frozen tumor biopsy specimen for extensive biomarker testing is taken before the start of treatment with a targeted agent included in the protocol. To patients who do not agree with tumor biopsy, this criterion does not apply. For children this will only be performed when needed as standard of care. Ability to understand and the willingness to sign a written informed consent document. |
||
| Key exclusion criteria | Ongoing toxicity from prior anti-cancer therapies > grade 2, other than alopecia. Patient is receiving any other anti-cancer therapy (cytotoxic, biologic, radiation, or hormonal other than for replacement) that is not part of the label (i.e. intended combination therapy is allowed). Specific combinations specified in the drug-specific label however will take precedence. Required wash out period prior to starting treatment within the protocol is at least two weeks. An exception is made for: Patients suffering from CRPC are allowed to continue androgen deprivation therapy. Medications that are prescribed for supportive care but may potentially have an anti-cancer effect (e.g., megestrol acetate, bisphosphonates). These medications must have been started ≥ 1 week prior to enrollment on this study. Patient is pregnant or nursing. Treatment with selpercatinib:
|
||
| Contact information | Log in voor de contactinformatie | ||